by Enoch Daniel
7 minutes
Pharma Manufacturing Trends To Watch In 2025: Smart Plants, Sustainability & Speed
In this article, we explore the details of this rapidly evolving field, uncovering the latest trends in pharmaceutical manufacturing.

Do you know that the pressure to develop new drugs that address unmet medical needs is more intense than ever before? After the COVID-19 pandemic outbreak, the pharmaceutical sector has grown to an immense height. With diseases evolving, patient demands increasing, and regulatory requirements becoming stricter, the need for innovation is paramount.
With each new challenge and opportunity, emerging trends and technologies offer promising solutions to longstanding problems. From streamlining production processes to enhancing drug efficacy and safety, these advancements can potentially grow the industry.
In this article, we explore the details of this rapidly evolving field, uncovering the latest developments and trends in pharmaceutical manufacturing shaping the future of medicine production.
What Is Meant By Pharmaceutical Manufacturing?
Pharmaceutical manufacturing is the process of producing drugs and medications on an industrial scale. It involves various processes from raw material sourcing to packaging finished products. These processes adhere to strict regulations and quality control measures to ensure the safety, efficacy, and consistency of the medications produced.
Pharmaceutical manufacturing facilities have specialized equipment and pharmaceutical technologies for mixing, granulation, compression, coating, and sterilization of various drugs. Moreover, the goal of pharmaceutical manufacturing is to efficiently produce continuous and high-quality medicines to meet regulatory standards and address the healthcare needs of patients worldwide.
What Are The Challenges Faced By Traditional Pharmaceutical Manufacturing Methods?
Traditional pharmaceutical manufacturing methods have been continuing for centuries and are equally essential in pharmaceutical manufacturing. However, they encounter several challenges amid the ever-evolving landscape of healthcare and technological innovation. Some of them are:
- Complexity & Resource Intensiveness: Traditional methods are complex and use hazardous materials which ultimately leads to high costs and longer production times. Also, traditional methods are more rigid and have limited flexibility in their process leading to rising hurdles in the drug manufacturing process.
- Environmental Impact: Traditional pharmaceutical manufacturing has a notable environmental impact, with high resource use, waste generation, and pollution contributing. All these lead to environmental degradation.
- Regulatory Compliance & Quality Control: Ensuring quality and safety while meeting regulatory standards is a challenge for traditional pharmaceutical manufacturing. Thus, increasing operational complexity.
What Are The Advantages Of Implementing Emerging Technologies In The Pharmaceutical Industry?
As we all know, embracing emerging pharmaceutical technologies in pharmaceutical manufacturing is crucial for competitiveness, progress, and shaping the future of pharmaceutical manufacturing. Here are key areas where modern solutions are making a significant impact:
- Accelerated Drug Discovery: Incorporating AI in drug discovery accelerates research and development, reduces costs, and fosters innovative therapies. This efficiency speeds up the transition from lab to market, providing patients with quicker access to new treatments.
- Data-Driven Decision-Making: Big data analytics enables informed decision-making in drug development, clinical trials, quality assessment, and reducing supply chain risks. This is achieved only when detailed data on suppliers' compliance and performance across markets is available.
- Cost Reduction: Data-driven decisions directly impact cost reduction. Adopting sustainable practices, like eco-friendly manufacturing and supply chain processes, lowers costs. Additionally, it meets corporate responsibility goals and reduces environmental impact.
- Market Expansion: When pharmaceutical companies embrace emerging pharmaceutical technologies, they attract top talent, foster collaboration, and build a reputation for innovation and excellence. Also, it helps in expanding healthcare services and reaching previously ignored populations.
Emerging Technologies In The Pharmaceutical Manufacturing Industry
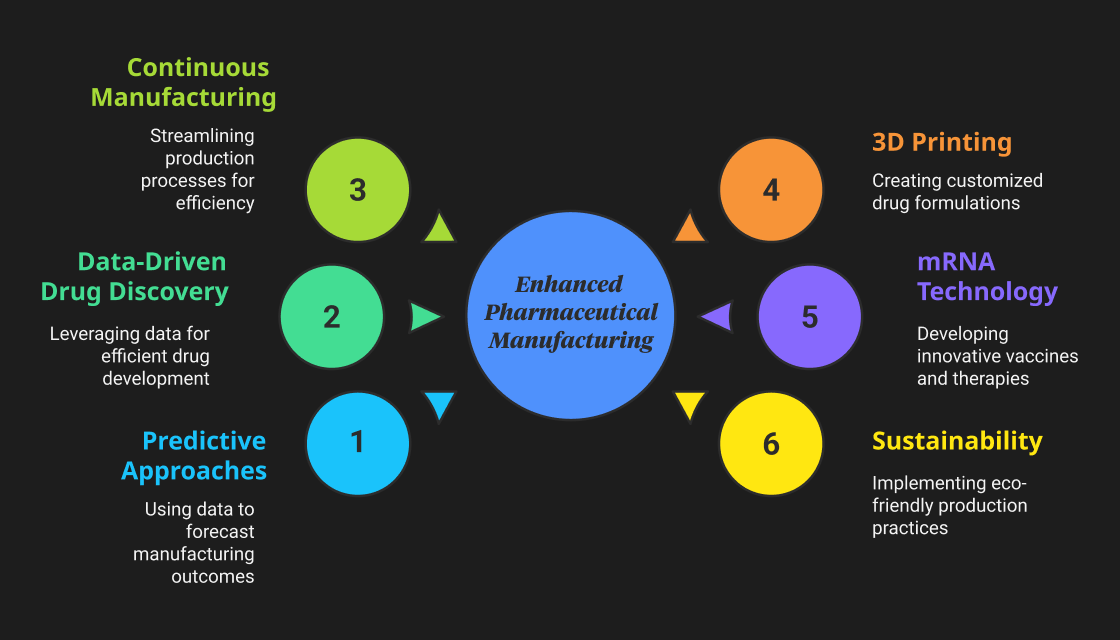
New technologies in pharmaceutical manufacturing are transforming drug development and production. Among these technological advancements, several technologies stand out as transformative tools. Let's learn more about these emerging technologies in pharmaceutical manufacturing and their impact on modern production lines.
1) Predictive Approaches
Predictive computational tools for solid formulation are revolutionizing pharmaceutical drug development. By predicting drug-excipient interactions and optimizing formulations early in the manufacturing process, these tools enhance the understanding of formulation mechanisms. This improved understanding leads to more efficient design and manufacturing processes, shortening development times and increasing patient access to effective therapies.
Additionally, these predictive approaches play a crucial role in modern production lines, including:
- Accelerates drug development timeline by enabling rapid virtual screening of potential formulations.
- Optimizes formulation parameters to enhance drug stability, bioavailability, and performance.
- Identifies potential issues early during the development process, allowing for proactive mitigation strategies.
- Reduces reliance on trial-and-error approaches, minimizing resource expenditure.
- Contributes to overall cost savings in drug development.
Some key predictive approaches include:
- In Trials: Conducts virtual clinical trials to predict outcomes and improve trial design without extensive human testing.
- Omics Technologies: Studies genetic, protein, and metabolic data to predict drug responses and potential side effects.
- Formulation Predictive Tools: Predicts how drugs will interact with other ingredients and what form they will take, affecting their stability and effectiveness.
- Real-World Data & Evidence: Uses patient records and real-world data to predict drug outcomes and identify safety issues.
2) Data-Driven Drug Discovery
Data is crucial in modern medicine across various domains, including diagnosis, treatment, research, and healthcare management. Data-driven drug discovery uses a vast array of data sources, from genomics to clinical records and chemical data, and accelerates the process by employing artificial intelligence and machine learning. This modern approach streamlines drug discovery, making it more efficient, cost-effective, and precise. It also accelerates the identification of potential drugs through systematic data collection and advanced algorithms.
Key components of data-driven drug discovery includes:
- Data Collection: Gathering genomic, clinical, chemical, biological, omics, and real-world data.
- Data Integration: Platforms harmonize and analyze diverse datasets for meaningful insights.
- Cheminformatics: Tools aid in compound identification and interaction analysis.
- Machine Learning: Algorithms extract features, predict interactions, and optimize drug development.
- Predictive Modeling: Identifies drug targets, screens compounds, assesses toxicity, and optimizes properties.
3) Continuous Manufacturing
Continuous manufacturing represents a framework for traditional batch manufacturing in pharmaceuticals. It is a method where drug products are produced without interruption, from raw materials to finished dosage forms, in a continuous flow. In this process, raw materials are continuously fed into the manufacturing line, and the final product is continually extracted, with real-time monitoring ensuring quality control throughout. Also, the approval of continuous manufacturing for Prezista by the FDA demonstrates regulatory acceptance of this approach, setting a precedent for future adoption.
The advantages of continuous manufacturing includes:
- Increased efficiency with uninterrupted processes, higher productivity, and reduced downtime.
- Less material waste due to constant operation and minimize losses.
- Real-time monitoring for enhanced quality control and assurance.
- Shorter cycle times and faster response to market demand.
- Greater scalability and easy adjustment to production volumes and demand.
4) 3D Printing In Drug Formulation
3D printing for personalized medicine has emerged as a groundbreaking application. It enables the precise fabrication of dosage forms tailored to individual patient needs. This approach revolutionizes drug delivery by allowing customized formulations that consider patient demographics, genetic variations, and specific treatment requirements. 3D printing enhances treatment efficacy and patient adherence by optimising dosage forms for each patient, ultimately improving therapeutic outcomes.
Moving forward, the potential impact of 3D printing on drug development and production extends beyond personalized medicine such as:
- 3D printing facilitates rapid prototyping and iteration of drug formulations. Thus allowing researchers to explore innovative drug delivery systems.
- With a 3D printing facility, researchers can optimize drug release profiles with exceptional details. It helps to tailor formulations to specific therapeutic requirements.
- The network design of 3D printing enables on-demand production, reducing lead times and enhancing supply chain efficiency.
- 3D printing technologies are getting better, offering cheaper and more adaptable ways to create complex medication forms.
5) mRNA Technology
Messenger RNA (mRNA) technology is a cutting-edge approach to medical intervention that harnesses the body's natural processes for protein production. mRNA serves as a blueprint for protein synthesis within cells, carrying instructions from DNA to ribosomes. This is where proteins are assembled. Moreover, this mRNA technology addresses critical medical challenges, including developing vaccines, gene editing, and protein therapy.
Traditionally, vaccine production has relied on weakened or inactivated pathogens to trigger an immune response. However, this approach accelerates vaccine development and enables rapid adaptation to emerging viral strains. Thus, quickly responding to infectious diseases.
Additionally, beyond vaccines, mRNA technology holds promise for several other things, like:
- Gene Editing: Corrects or modifies faulty genes for genetic disorders.
- Protein Therapies: Delivers therapeutic proteins for enzyme or hormone deficiencies.
- Cancer Immunotherapy: Targets tumor antigens for immune system activation against cancer.
6) Sustainability In Production
Sustainability in pharmaceutical production refers to minimizing the environmental impact of manufacturing processes while maintaining product quality and efficiency. With the help of emerging technologies, the industry is making significant strides toward greener operations.
One major shift is the move from traditional batch processing to continuous manufacturing. Continuous systems are more energy-efficient, produce less waste, and consume fewer raw materials. For example, the FDA has endorsed continuous manufacturing for its ability to reduce production footprints and improve environmental outcomes.
Sustainability in production also includes:
- Green Chemistry: It is also gaining traction, focusing on designing processes that use safer solvents, reduce hazardous by-products, and optimize resource use. Companies like GSK and Pfizer have adopted greener synthetic routes to lower emissions and reduce water consumption.
- Digital Technologies & AI: Another critical area is the use of digital technologies and AI. These tools allow real-time monitoring of manufacturing lines, reducing energy use, preventing overproduction, and minimizing discarded materials by catching errors early.
- Single Use Systems: single-use systems in biologics production reduce water and energy used in sterilization, though efforts are ongoing to make these systems more recyclable or biodegradable to address plastic waste concerns.
In essence, sustainability in production is no longer optional, it’s a necessity. Emerging technologies are enabling pharmaceutical companies to meet regulatory expectations, reduce environmental impact, and support long-term global health goals.
What Are The Latest Trends In Pharmaceutical Manufacturing?
As the pharmaceutical industry advances, staying ahead of the curve of the latest trends promoting manufacturing processes is crucial. Let's delve into the key trends in pharmaceutical manufacturing driving innovation in pharmaceutical manufacturing.
A) Process Modeling
Process modeling uses computational techniques like Computational Fluid Dynamics (CFD), Discrete Element Method (DEM), and Population Balance Models (PBM) to simulate pharmaceutical processes. This offers insights and enables continuous manufacturing. Also, regulatory bodies like the FDA endorse process modeling for its role in enhancing quality and compliance in pharmaceutical manufacturing.
The benefits of process modeling are:
- Refine Process Steps: Process modeling enhances each manufacturing step's efficiency by identifying and addressing inefficiencies and customizing steps for different drugs.
- Economize Operations: Optimizing processes reduces waste, minimizes resource use, and lowers production costs, contributing to a smaller environmental footprint.
- Validate Assumptions: Testing assumptions in virtual environments reduces risk and speeds up development. And, minimizing the need for physical trials.
- Identify Critical Process Parameters: Models highlight parameters that impact quality and performance, enabling their optimization for yield, purity, and consistency.
- Advance Manufacturing Paradigm: Process modeling drives innovation, improves efficiency, reduces costs, enhances product quality, and ensures regulatory compliance.
B) System Biotechnology
Over recent decades, biochemical science has evolved from a single-gene focus to understanding complex gene interactions within cells. This shift led to systems biology, which explores complex gene networks using advanced computational and experimental techniques for holistic understanding.
This helps researchers in understanding cellular functions and interactions, benefiting the pharmaceutical in multiple ways, including:
- Identification of Drug Targets: Analyzing gene and protein networks identifies critical points for therapeutic intervention, increasing drug development success rates.
- Optimization of Drug Efficacy & Safety: Detailed insights into drug interactions help predict side effects early, ensuring safer and more effective drugs.
- Enhanced Biomarker Discovery: Systems biology identifies patterns for new biomarkers, aiding in disease diagnosis, progression prediction, and treatment monitoring.
- Improved Bioprocess Development: Understanding metabolic pathways optimizes production processes, enhancing efficiency, yield, and cost-effectiveness in drug manufacturing.
- Integration With Computational Tools: Systems biology integrates with computational tools to simulate biological processes, refine parameters, and optimize production workflows.
C) Personalized Medicine
Personalized medicine has its importance while recognizing each patient is unique and may respond differently to the same medication due to genetic variations, metabolic differences, or other factors. By integrating their characteristics into treatment plans, treatments are tailored to each patient's specific needs and circumstances, offering a more targeted and practical approach to healthcare.
Moreover, the implications of personalized medicine for manufacturing processes are significant, as they require a shift from traditional mass-production models to more flexible and adaptable manufacturing systems.
Some key implications includes:
- Flexible Manufacturing: Personalized medicine often involves producing smaller batches of drugs tailored to individual patients. This necessitates flexible manufacturing processes capable of quickly adjusting production schedules and accommodating diverse formulations.
- Customized Formulations: Manufacturing facilities must be equipped to produce a wide range of customized formulations to meet the unique needs of patients. This involves using advanced technologies like 3D printing to create bespoke dosage forms.
- Data Integration: Manufacturing processes must be integrated with patient data systems to ensure that treatments are accurately matched to individual patients. This requires robust data management systems capable of securely handling sensitive patient information.
D) Artificial Intelligence (AI) & Machine Learning
Artificial intelligence (AI) is becoming increasingly prevalent in pharmaceutical organizations, offering valuable assistance in various drug discovery and manufacturing aspects. Through AI-powered algorithms, pharmaceutical companies can accelerate the process of identifying potential drug candidates, optimize manufacturing processes, and create effective strategies for post-launch activities such as marketing and distribution.
Primary applications of AI in drug discovery and development include:
- Drug design: AI-powered platforms predict the molecular structure of potential drug candidates, optimizing their efficacy and reducing development timelines.
- Virtual Screening: AI models screen large databases of compounds to identify those with the highest likelihood of binding to target molecules, speeding up the identification of lead compounds.
- Repurposing Existing Drugs: AI algorithms identify new therapeutic uses for existing drugs by analyzing their molecular properties and biological effects.
Future Directions In Pharmaceutical Manufacturing
Continuous research and innovation are shaping the future of pharmaceutical manufacturing. These advancements promise to revolutionize drug production, enhance therapy, and tackle healthcare challenges, helping the industry meet evolving patient and system needs globally.
With the evolution of pharmaceutical manufacturing come new technologies. Here, we explore the future of pharmaceutical manufacturing that will define the future of the pharma industry.
- Biopharmaceuticals: The rise of biopharmaceuticals, including monoclonal antibodies and gene therapies, will lead to the adoption of specialized manufacturing processes. These processes, often involving cell culture-based production systems, require advanced manufacturing facilities and expertise.
- Digitalization & Automation: Digitalization and automation will streamline manufacturing processes, improve productivity, and ensure regulatory compliance. Technologies like artificial intelligence (AI) and the Internet of Things (IoT) enable real-time monitoring and predictive maintenance, minimizing downtime and optimizing resource utilization.
- Advanced Analytics & Predictive Modeling: Pharmaceutical manufacturers will increasingly rely on advanced analytics to optimize production processes, forecast demand, and prevent quality issues. These data-driven approaches enable proactive decision-making, improving efficiency and cost savings.
- On-Demand Manufacturing: The concept of on-demand manufacturing, enabled by technologies like 3D printing and continuous manufacturing, will gain traction. This approach allows for flexible and responsive production, reducing the need for extensive inventories and enabling faster response to market demands and supply chain disruptions.
- Collaboration & Outsourcing: Pharmaceutical manufacturers will increasingly collaborate with contract development and manufacturing organizations (CDMOs) to access specialized expertise, technologies, and production capacity. This trend allows companies to focus on core competencies, accelerate time to market, and mitigate risks associated with capital-intensive manufacturing investments.
What Are The Potential Challenges And Opportunities?
While these advancements hold tremendous promise, they also present some challenges that must be addressed:
- Regulatory Compliance: Compliance with evolving regulatory standards adds complexity to technological adoption in the highly regulated pharmaceutical industry, with data integrity posing an additional challenge. Regulatory agencies must adapt their frameworks to accommodate emerging technologies in pharmaceutical manufacturing while ensuring product safety and efficacy.
- Data Privacy: Given the reliance on digital solutions and handling sensitive information, data privacy and security are paramount to protect against cyber threats and safeguard sensitive information. It also helps maintain trust with stakeholders, including patients, healthcare providers, and regulatory authorities.
- Initial Investment Cost: While technology offers efficiency gains, initial investment and ongoing operational costs can be burdensome, necessitating a balance between innovation and cost management. Companies must continually update their infrastructure or collaborate with third-party tech firms for accelerated processes.
- Skilled Workforce: Innovation demands a skilled workforce proficient in advanced manufacturing techniques and digital tools. Continuous training is essential to empower workers with the skills needed for success in pharmaceutical manufacturing, ensuring they can effectively use new technologies and contribute to sustained growth.
- Supply Chain Resilience: The COVID-19 pandemic exposed vulnerabilities in pharmaceutical supply chains, emphasizing the need for resilience and flexibility in manufacturing operations to ensure continued access to essential medications and medical supplies globally.
In Conclusion
The pharmaceutical industry is rapidly changing due to new trends and technologies. While innovations like process modeling and personalized medicine offer great potential, challenges such as meeting regulations, protecting data, and managing costs remain.
Companies must balance innovation with cost management, prioritize resilience, and invest in workforce training to succeed.
By embracing the new principles and using the power of collaboration and emerging technologies, the pharmaceutical industry can ensure its readiness to meet evolving patient needs and regulatory requirements in a rapidly changing healthcare landscape.
FAQs
1) What’s Driving Innovation In Pharma Manufacturing?
Innovation in pharma manufacturing is driven by new technologies like automation, artificial intelligence, and continuous manufacturing. These help make production faster, more efficient, and more cost-effective. Growing demand for personalized medicine and stricter regulations also push companies to find better and smarter ways to make medicines.
2) How Are Manufacturers Balancing Speed And Quality?
Manufacturers balance speed and quality by using advanced technologies and digital tools that allow them to monitor and control production in real-time. They follow strict quality standards while streamlining processes to avoid delays. This way, they can deliver safe, high-quality medicines without compromising on speed.





